Kristi Leigh TV – Feb 15 2022
Former Blackrock Portfolio Manager Edward Dowd Exposes Pfizer Fraud

Excerpt: We’re On Team Humanity:
Covid-19: Researcher blows the whistle on data integrity issues in Pfizer’s vaccine trial
BMJ – Paul D Thacker reporting – Nov 02 2021
Revelations of poor practices at a contract research company helping to carry out Pfizer’s pivotal covid-19 vaccine trial raise questions about data integrity and regulatory oversight.
In autumn 2020 Pfizer’s chairman and chief executive, Albert Bourla, released an open letter to the billions of people around the world who were investing their hopes in a safe and effective covid-19 vaccine to end the pandemic. “As I’ve said before, we are operating at the speed of science,” Bourla wrote, explaining to the public when they could expect a Pfizer vaccine to be authorised in the United States.1
But, for researchers who were testing Pfizer’s vaccine at several sites in Texas during that autumn, speed may have come at the cost of data integrity and patient safety. A regional director who was employed at the research organisation Ventavia Research Group has told The BMJ that the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase III trial. Staff who conducted quality control checks were overwhelmed by the volume of problems they were finding. After repeatedly notifying Ventavia of these problems, the regional director, Brook Jackson, emailed a complaint to the US Food and Drug Administration (FDA). Ventavia fired her later the same day. Jackson has provided The BMJ with dozens of internal company documents, photos, audio recordings, and emails.
Poor laboratory management
On its website Ventavia calls itself the largest privately owned clinical research company in Texas and lists many awards it has won for its contract work.2 But Jackson has told The BMJ that, during the two weeks she was employed at Ventavia in September 2020, she repeatedly informed her superiors of poor laboratory management, patient safety concerns, and data integrity issues. Jackson was a trained clinical trial auditor who previously held a director of operations position and came to Ventavia with more than 15 years’ experience in clinical research coordination and management.
In trying to tackle fake news, Facebook is cracking down on real science
The platform’s fact checkers found no inaccuracies in a recent BMJ investigation – but limited its reach anyway. Should they be playing moral police?
The New Statesman – Jan 29 2022 By Rebecca Coombes and Madlen Davies

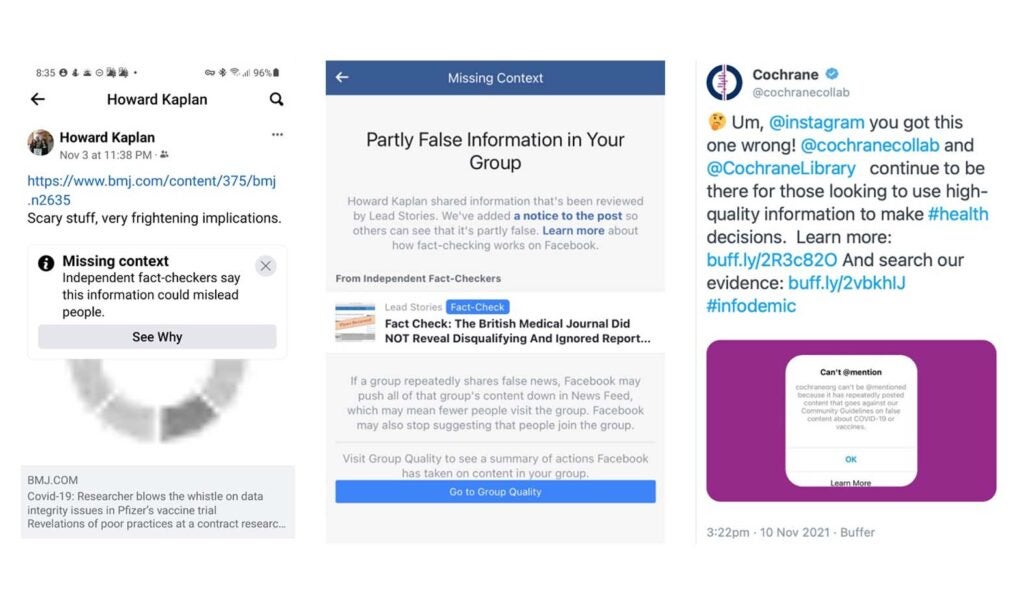
On 3 November, Howard Kaplan, a retired dentist from Israel, posted a British Medical Journal (BMJ) investigation to a private Facebook group. The article reported poor practices occurring at Ventavia, a research company contracted to run three trial sites for the Pfizer Covid-19 vaccine.
The article brought record traffic to bmj.com, and was widely shared on Twitter. But a week later, Kaplan woke up to a message from Facebook. “The Facebook Thought Police has issued me a dire warning,” he posted. “Facebook’s ‘independent fact-checker’ doesn’t like the wording of the article by the BMJ. And if I don’t delete my post, they are threatening to make my posts less visible… If it seems like I’ve disappeared for a while, you’ll know why.” Other BMJ readers also reported problems sharing the story.
There are now around 300 fact-checking organisations across the world, many of them fledgling companies with small budgets. Facebook in particular bestows a great deal of authority upon its 80 third-party fact checkers. But our recent experience at the BMJ, an established global publication, is that fact-checking can be incompetent, irresponsible and capable of suppressing already fully sourced and peer-reviewed journalism.
As well as warning Kaplan and others not to share the story, the BMJ investigation was given a Facebook “Missing Context” label. (These warn that “this information could mislead people”.) Readers were directed to a “fact check” article by Lead Stories, one of seven companies contracted by Facebook in the US, whose tagline is “debunking fake news as it happens”. (According to an analysis last year, Lead Stories was responsible for half of all Facebook fact checks.)